
objective response rate (ORR) according to Response
Evaluation Criteria in Solid Tumors (RECIST)
[22] .Secondary
outcomes included adverse events (AEs) and efficacy analyses
according to PD-L1 expression status in tumor tissue.
2.3.
Data extraction
Two independent reviewers (M.R. and A.A.M.V.) assessed
relevant articles for study eligibility, and any disagreement
on inclusion was resolved by discussion. Using a standard-
ized data extraction form, the following details were
extracted: study design, number of patients, patient
characteristics, treatment intervention, median duration
of follow-up, survival data, ORR, AEs, and PD-L1 expression
status. Data were extracted from all included studies by one
reviewer (M.R.) and subsequently checked by a second
reviewer (A.A.M.V.) to ensure their accuracy.
2.4.
Data analysis
Descriptive analyses were used to present the data.
Continuous outcomes were described using mean and
standard deviation, or alternatively, median and (inter-
quartile) range. For categorical outcomes, frequencies and
proportions were used. If reported, hazard ratios (HRs) with
confidence intervals (CIs) were mentioned. Owing to the
limited number of available studies, no quantitative
analysis (ie, meta-analysis) could be performed.
3.
Evidence synthesis
3.1.
Study selection
The initial literature search identified 3354 articles. After
removing duplicate studies, one reviewer (M.R.) evaluated
all titles and abstracts. Finally, 40 publications were
identified as potentially relevant and were retrieved for
full-text evaluation. According to the inclusion criteria, six
randomized phase 1–3 clinical trials were selected for
evidence synthesis (one trial on UCC, three trials on RCC, and
two trials on PC;
Table 1 ). The literature search identified
16 additional non-RCTs addressing the safety and efficacy of
ICIs in urological cancer (Supplementary Tables 1 and 2).
3.2.
Characteristics, efficacy, and PD-L1 status in selected
studies
The characteristics, efficacy measures, and PD-L1 status of
the included studies are presented in
Tables 1, 2, and 3,
respectively (see also Supplementary Table 3).
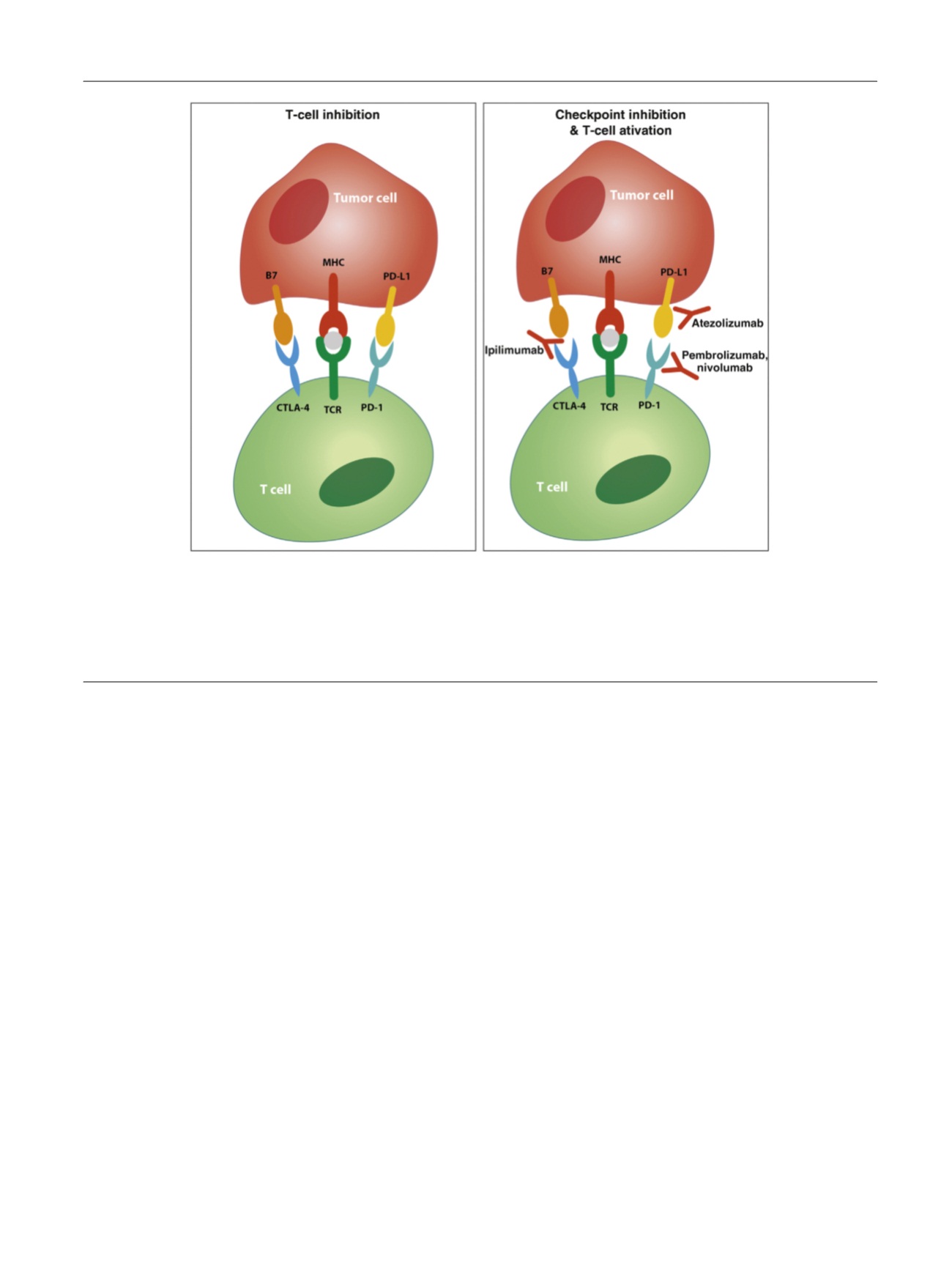
[(Fig._1)TD$FIG]
Fig. 1 – T-cell coinhibitory receptor expression and checkpoint inhibition. Tumor cells and antigen presenting cells (APCs) express a specific antigen
that is presented to cytotoxic T cells in a peptide major histocompatibility complex (MHC). T cells recognize this presented antigen with their T-cell
receptor (TCR) and, together with binding of costimulatory receptors (eg, CD28); this leads to T-cell activation and subsequently elimination of the
(tumor) cell. Interaction of coinhibitory receptors on T cells with their ligands on APCs or tumor cells inhibits T-cell activation. Known coinhibitory
receptors are PD-1 (that interacts with its ligand PD-L1) and CTLA-4. Blocking antibodies against these coinhibitory receptors or their ligands can
prevent their interaction and the subsequent inhibition of T-cell activity. CTLA-4 = cytotoxic T lymphocyte–associated protein 4; PD-1 = programmed
cell death 1; PD-L1 = programmed cell death receptor ligand 1.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 4 1 1 – 4 2 3
413
















