
a range of plausible cost and benefit ratios
( Fig. 1and
Supplementary Fig. 3).
The clinical consequences, or the number of biopsies and
the number of high-grade cancers that could be avoided or
delayed per 1000 patients, were illustrated based on
prediction models with the 4Kpanel or PSA
( Table 5 ). For
example, using a model with the 4Kpanel and a clinical rule
of only performing an initial surveillance biopsy in patients
whose risk of high-grade cancer exceeded 10%, 252 biopsies
would be avoided, 19 of which would contain high-grade
cancer as defined by any pattern 4 disease, and zero biopsies
with primary Gleason 4. Comparing the two models at the
same numbers of biopsies avoided (Supplementary Fig. 4)
shows that the 4K model appears to miss fewer higher-
grade cancers while avoiding the same number of initial
biopsies.
4.
Discussion
In this study using a prospectively enrolled multi-institu-
tional cohort of men on active surveillance, we show that
addition of a panel of four kallikrein markers to a model that
includes clinical information can significantly improve
prediction of the outcome in the first surveillance biopsy.
Both models performed comparably for prediction of
reclassification in subsequent biopsies. Importantly, in
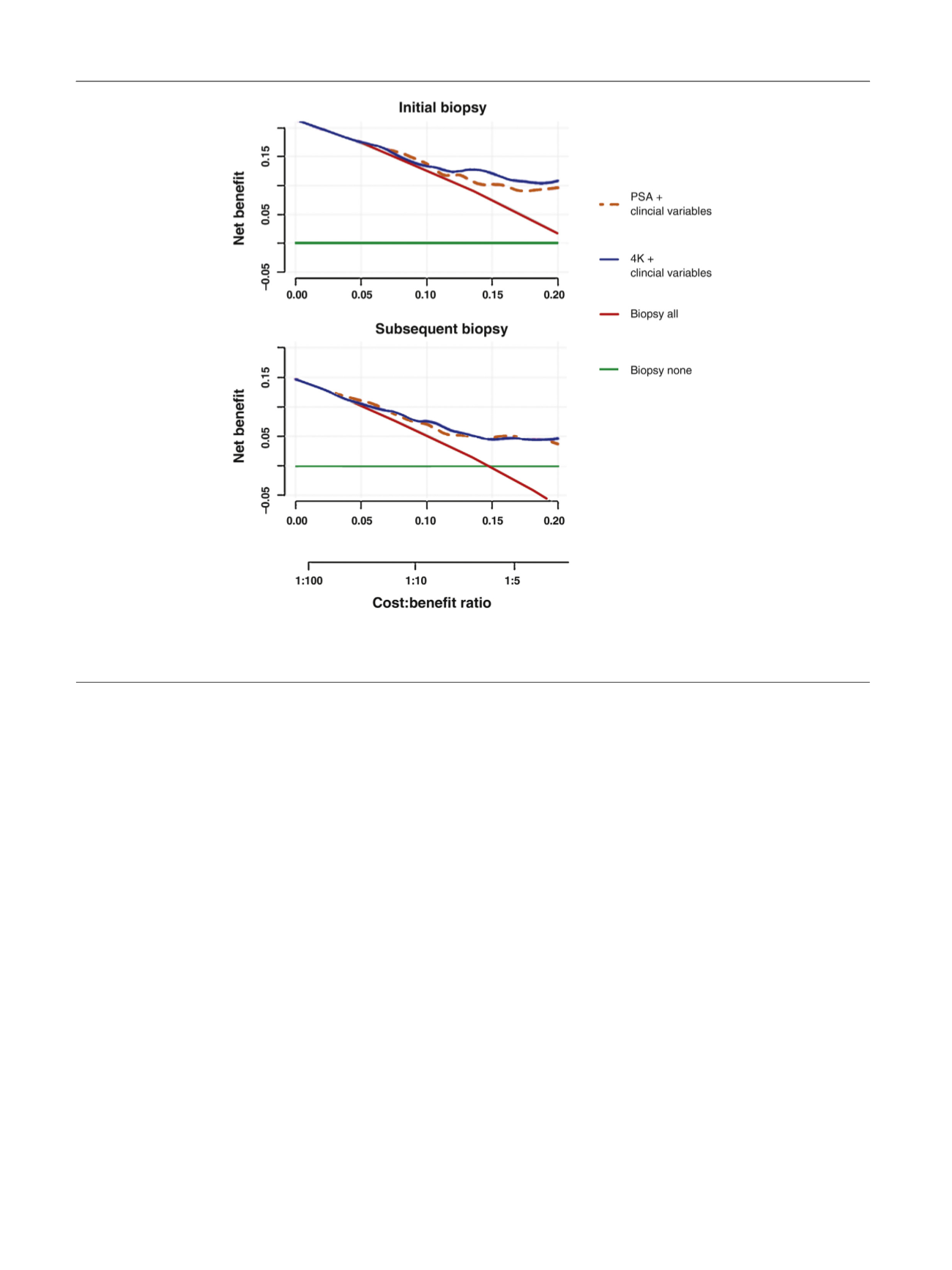
DCA both models showed a higher net benefit compared
to biopsy-all and biopsy-none strategies. Lastly, we showed
that the 4Kpanel added to currently available clinical
metrics and how the results impact clinical management.
There is a growing body of evidence that true Gleason
6 prostate cancer is indolent and will not cause harm if left
untreated
[10–12] .This knowledge is balanced by the
known undersampling in prostate needle biopsies, and
while some have advocated that select Gleason 3 + 4 cancers
may undergo surveillance, level 1 clinical trial data and
treatment guidelines generally recommend treatment of
higher-grade cancers, including Gleason 3 + 4 disease
[13,14]. Our efforts focus on developing tools for use after
diagnosis of Gleason 6 prostate cancer to provide a higher
degree of certainty that no occult high-grade cancer was
missed at diagnosis. More accurate tools would not only
support the practice of active surveillance but could also
promote less intensive monitoring regimens.
A panel of four kallikreins, when combined in a
mathematical algorithm, improves the prediction of newly
diagnosed high-grade (Gleason 7) cancer
[3]. This panel of
markers also improved long-term prediction of metastatic
disease among men with PSA 2 in a Swedish cohort
[15]. In
this study, we asked whether the same panel of markers
[3]improved the prediction of high-grade disease in surveil-
lance biopsies of men already diagnosed with Gleason
[(Fig._1)TD$FIG]
Fig. 1 – Decision curve analysis for full models with serum Prostate-specific antigen (PSA) or with the 4Kpanel. Strategies for biopsying all men (biopsy
all) or no men (biopsy none) are also shown. The line with the highest net benefit at any particular threshold probability for biopsy (
x
-axis) will yield
the best clinical results.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 4 4 8 – 4 5 4
452
















