
1.
Introduction
Although radical prostatectomy (RP) is associated with
excellent oncologic results in patients with localized
prostate cancer (PCa), up to 15% of men treated with this
approach experience clinical recurrence (CR) at long-term
follow-up
[1] .Over the last few years the introduction of
novel imaging modalities with high sensitivity at low
prostate-specific antigen (PSA) levels allowed for a prompt
identification of the site of recurrence after primary
treatment
[2–6]. This is crucial, since patients affected by
nodal metastases only showed better prognosis compared
with their counterparts with skeletal or visceral metastases
[2,3,7–9]. These observations led to the hypothesis that men
with an exclusive involvement of the lymph nodes at the
time of recurrence might be affected by a less aggressive
disease and, therefore, could benefit from metastases-
directed therapies aimed at maximizing local control
[10–13].
Several retrospective series reported that salvage lymph
node dissection after primary treatment might be an option
associated with acceptable oncologic outcomes in patients
with nodal recurrence at PET/CT scan. In particular, this
surgical approach would prolong CR-free survival and defer
the use of systemic therapies
[11,14–17]. Recently, the
possible value of this therapeutic option has been also
reported by the European Association of Urology Guidelines
on Prostate Cancer
[18]. Nonetheless, available studies are
based on patients treated with the open approach and
evidence is scarce regarding the safety and effectiveness of
minimally invasive techniques in this setting. Under this
light, we aimed at reporting perioperative, pathologic, and
oncologic outcomes of robot-assisted salvage nodal dissec-
tion (RASND) in patients with recurrence limited to the
pelvic and/or retroperitoneal lymph nodes after RP docu-
mented by positron emission tomography/computed to-
mography (PET/CT) scan treated at two high-volume
robotic centers.
2.
Materials and methods
2.1.
Patient population
After ethical committee approval, 16 patients with postoperative
biochemical recurrence (BCR; defined as two consecutive PSA values
>
0.2 ng/ml) after RP and nodal uptake at choline (
n
= 3) or prostate-
specific membrane antigen (PSMA;
n
= 13) PET/CT scan suggesting the
presence of a nodal recurrence were retrospectively identified. All
patients were evaluated with abdominal CT scan to exclude the presence
of other visceral or skeletal metastatic sites. Patients were treated with
RASND by two experienced robotic surgeons at two high-volume
European centers between June 2015 and April 2016.
2.2.
Surgical technique
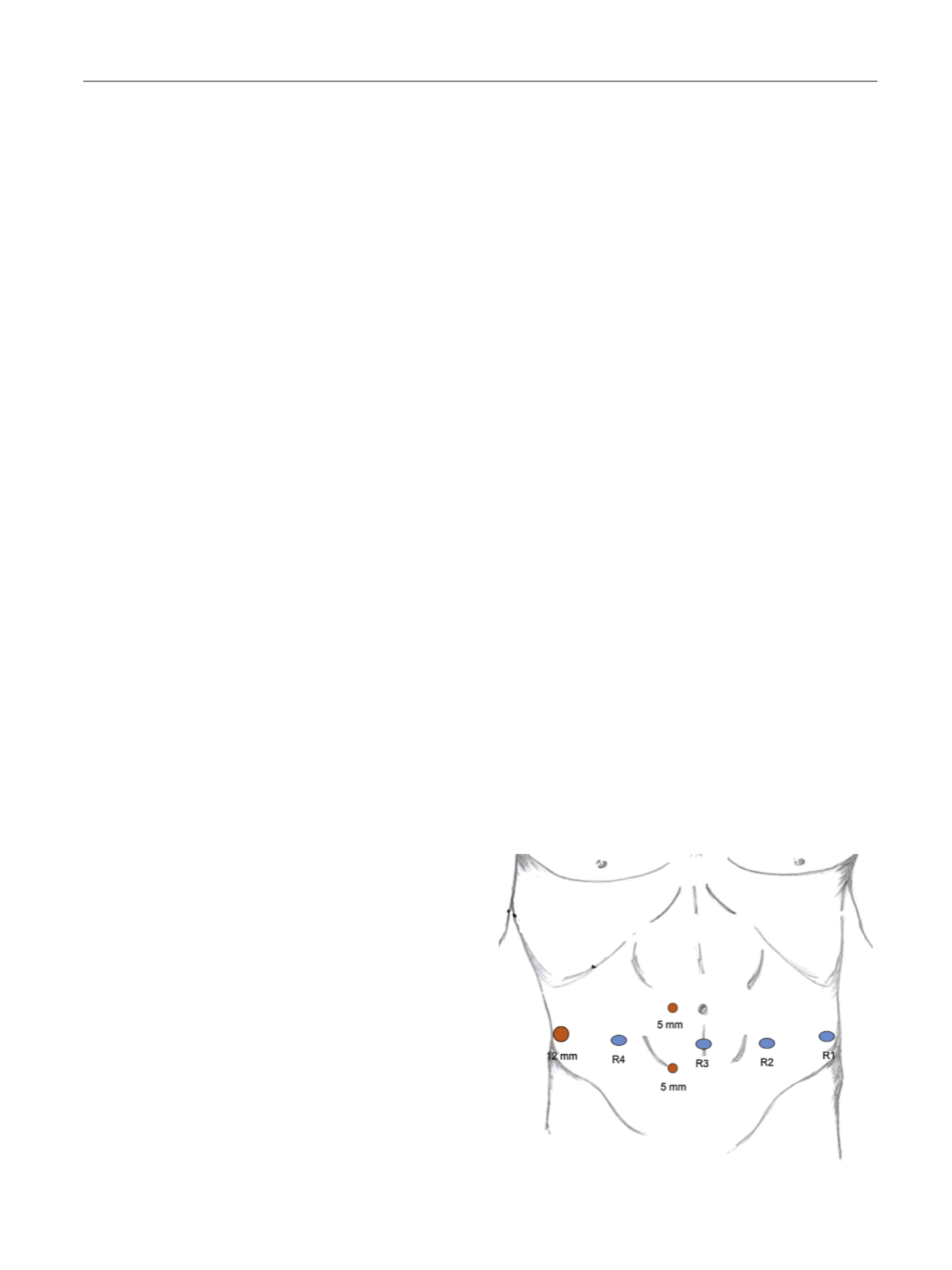
All procedures were performed through a six-port transperitoneal
approach using the four-arm Da Vinci Si (
n
= 3) or Xi (
n
= 13) Surgical
Systems (Intuitive Surgical, Sunnyvale, CA, USA). The following robotic
instruments were used: (1) monopolar scissors, (2) fenestrated bipolar
forceps or Maryland bipolar forceps, (3) prograsp forceps, and (4) large
needle driver. After induction of general anesthesia, the patient was
placed in the lithotomy position at a steep Trendelemburg position,
allowing the bowel to fall cephalad. A single preoperative dose of
antibiotic prophylaxis was administered. The camera trocar was placed
3 cm below the umbilicus. Two 8-mm robotic trocars were placed
bilaterally at 8 cm from the camera port. Another 8-mm robotic trocar
was placed on the same line of the camera trocar at 8 cm from the other
robotic port. One AirSeal valveless 12-mm assistant trocar (Surgiquest,
Milford, CT, USA) was placed 3 cm above the iliac crest on the opposite
side. Two additional 5-mm assistant trocars were placed between
the camera port and the robotic port 2 cm above and below the line of
the robotic trocars
( Fig. 1 ). The da Vinci surgical systemwas then docked
in the pelvic configuration.
After localization of the external iliac vessels, the peritoneum was
incised and dissection of the lymphatic tissue was performed. Pelvic
RASND initially consisted of excision of all fibrofatty tissue along the
external iliac vessels, with the distal limit being the deep circumflex vein
and the femoral canal. All fibrofatty tissue within the obturator fossa was
removed and the obturator nerve was completely skeletonized. The
lateral limit consisted of the pelvic sidewall and medially the dissection
limit was represented by the perivesical fat. The Marcille’s triangular
lumbosacral fossa was also dissected free. This area is defined laterally
by the medial border of the psoas, medially by the body of the fifth
lumbar vertebra and inferiorly by the sacral wing
[19]. Proximally
RASND included removal of all lymph nodes along common iliac vessels
up to the aortic bifurcation
( Fig. 2 ). The ureters were identified and
isolated bilaterally. The lymph nodes located laterally and medially to
the internal iliac artery were removed. The presacral nodes were then
dissected bilaterally. At the end of the procedure the ureters and the iliac
vessels were completely skeletonized up to the aortic bifurcation
( Fig. 3 ).
Retroperitoneal RASND was performed in 13 (81.3%) patients.
Following completion of the pelvic nodal dissection, the robot was
dedocked, rotated 180
8
, and then redocked. In patients treated with the
Da Vinci Si surgical system, this step was accomplished by repositioning
the robot parallel to the left leg. With the Xi system the arms were
disconnected and the boom was rotated 180
8
. The posterior layer of the
peritoneum was incised at the level of the aortic bifurcation and the
retroperitoneal space was entered. The third robotic arm was used for
traction of the posterior layer of the peritoneum, facilitating the access to
the retroperitoneal space. Dissection was then carried out cranially. The
inferior mesenteric artery was identified and isolated
( Fig. 4 ). All nodal
tissue located between the aortic bifurcation and the renal vessels was
[(Fig._1)TD$FIG]
Fig. 1 – Port placement for the robot-assisted salvage nodal dissection.
E U R O P E A N U R O L O G Y 7 2 ( 2 0 1 7 ) 4 3 2 – 4 3 8
433
















